Learning the kinds and causes of corrosion, and techniques on how to prevent, control and mitigate it is the first step in combating this process. A variety of resources are avaialable for teachers and the public from the DoD Corrosion Policy and Oversight Website. 
Protocol 424 Do you have what it takes to fight Corrosion? Find out here as you take on Protocol 424; an e-learning game where you will build, repair, and maintain infrastructures within an old R&D base.
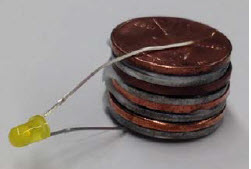
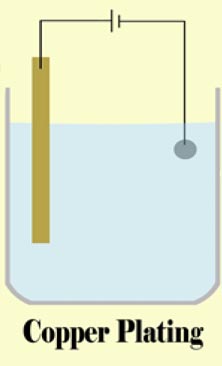
Stack metals togrther to make a battery. In this demo we are forcing the ANODIC reaction of iron corrosion by pulling electrons out of the iron and “pumping” them over to the graphite rod where it allows a CATHODIC reaction of that converts water to hydrogen and GAINS electrons in the process. The overall reaction would go spontaneously but we are making it go faster with a battery (an electron pump). 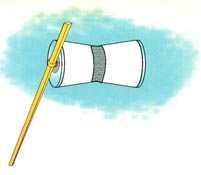
Energy is neither created nor destroyed it is only transformed. Kinetic and potential energy are often paired together in simple demonstrations like a Rubber Band Car. |
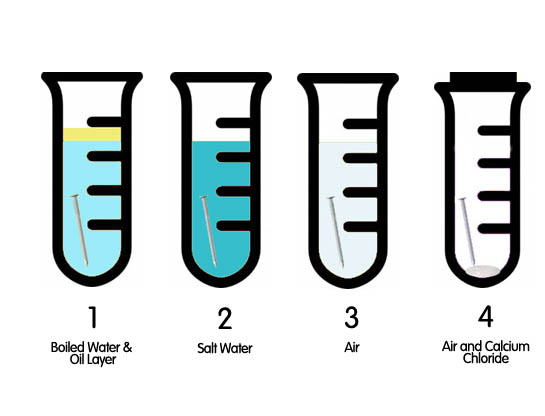
Definition Corrosion is the chemical reaction where metals break down slowly because of other elements in their environment. For example when you see a rusty car or bridge, corrosion is the cause. Rusting, a well known example of corrosion, is the breakdown of the metal iron. Introduction Every day bridges, automobiles, aircraft, and hundreds of other items made of metal corrode bit by bit. Produced for the DoD Corrosion Office by Bruno White Entertainment as part of a comprehensive series of videos about corrosion,LaVar Burton describes the degradation process through video clips and visual images. This video would be a great introduction to any lesson involving corrosion. Corrosion Investigation (Worksheet pdf file) Problem What are the three conditions that cause iron to rust? Materials 1. Four Test Tubes Procedure Place an iron nail or iron shavings in each of the test tubes along with the designated ingredient(s). Place the test tubes on a shelf. Check in 24-48 hours to see if any rust has formed. Report your results on the worksheet.
|
||||||||||||||||||||
|
|||||||||||||||||||||
© 1996 - 2016 Linda C. Joseph
All Rights Reserved
All CyberBee Graphics are Trademarked
Graphics by
Darlene Vanasco/Creative Director
Erika Taguchi/Designer & Illustrator
Hosting Provided by Iwaynet